In the intricate realm of molecular biology and biochemistry, polymeric nucleic acids serve as the fundamental molecules encoding genetic information. Their pivotal role extends from the central dogma of molecular biology—DNA replication and transcription—to cutting-edge applications in biotechnology, medicine, and nanotechnology. For professionals engaged daily with nucleic acid research or therapeutic development, comprehending the nuanced concepts, innovative applications, and emerging breakthroughs surrounding polymeric nucleic acids can markedly influence experimental design and translational strategies. This article delves into the core principles governing polymeric nucleic acids, explores their diverse applications with concrete examples, and highlights recent technological innovations shaping the future of nucleic acid science.
Core Concepts of Polymeric Nucleic Acids: Structure, Function, and Synthesis

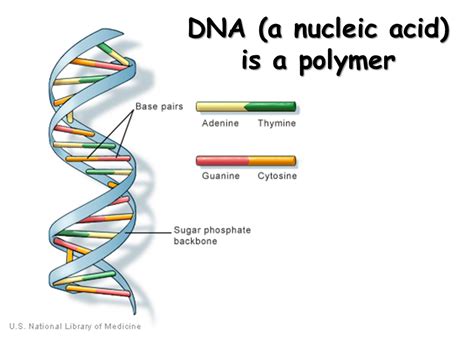
At their essence, polymeric nucleic acids—primarily deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)—are biopolymers composed of nucleotide monomers linked via phosphodiester bonds. Each nucleotide consists of a nitrogenous base, a sugar moiety (deoxyribose in DNA and ribose in RNA), and a phosphate group, forming the backbone of the molecule. The sequence of these bases encodes genetic instructions essential for cellular function and heredity.
Understanding the structural distinctions between DNA and RNA is foundational for appreciating their functionality. DNA adopts a stable double-helical structure characterized by complementary base pairing—adenine with thymine, and guanine with cytosine—facilitated by hydrogen bonds. RNA, in contrast, often exists as a single strand with versatile secondary structures, including loops and bulges, enabling diverse regulatory roles.
Synthesis of polymeric nucleic acids in nature occurs through enzymatic processes such as DNA polymerase-driven replication and RNA transcription. In laboratory settings, chemical synthesis of oligonucleotides employs phosphoramidite chemistry, which allows precise sequence assembly of synthetic nucleic acids up to several hundred bases long, facilitating research and therapeutic applications.
| Relevant Category | Substantive Data |
|---|---|
| Synthesis Method | Phosphoramidite chemistry achieves high-fidelity oligonucleotide assembly with >99% coupling efficiency |
| Stability | DNA exhibits greater chemical stability (+stronger backbone resistance to hydrolysis), whereas RNA’s 2’-OH group imparts susceptibility to alkaline hydrolysis |
| Size Limit | Synthetically produced oligonucleotides typically range up to 200 bases, with longer sequences assembled via enzymatic or ligation methods |

Applications of Polymeric Nucleic Acids in Modern Science and Medicine

The versatility of polymeric nucleic acids extends beyond their genetic functions, deeply embedding into diverse technological domains. From targeted gene regulation to nanomaterial construction, their multifaceted utility hinges on specific properties like hybridization specificity, programmability, and biocompatibility.
Gene Therapy and Precision Medicine
One of the most transformative applications of synthetic nucleic acids is in gene therapy. Oligonucleotides such as antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and microRNAs (miRNAs) are designed to modulate gene expression by binding complementary sequences to either inhibit mRNA translation or induce degradation via RNA-induced silencing complexes (RISCs). Recent approvals—such as nusinersen for spinal muscular atrophy—highlight the clinical impact of these molecules.
Advances in chemical modification have enhanced nuclease resistance and biodistribution, critical for effective delivery. Notably, lipid nanoparticle (LNP) vectors have revolutionized siRNA delivery, exemplified by the COVID-19 mRNA vaccines that employ LNP encapsulation for efficient cellular entry.
| Relevant Category | Substantive Data |
|---|---|
| Clinical Development | Over 20 nucleic acid-based therapeutics approved globally as of 2023, targeting diseases from genetic disorders to cancers |
| Delivery Efficiency | LNP technology achieves >90% delivery rate in hepatocytes, with ongoing research into tissue-specific vectors |
| Stability Enhancement | Chemical modifications can extend serum half-life from 30 minutes to over 48 hours in vivo |
Diagnostics and Biosensing Technologies
Polymeric nucleic acids underpin modern diagnostic assays, including quantitative PCR (qPCR), digital PCR, and hybridization-based biosensors. For instance, aptamers—synthetic nucleic acid ligands—exhibit high affinity and specificity for target proteins, enabling rapid, label-free detection in disease diagnostics.
CRISPR-Cas systems utilize guide RNA (gRNA) sequences as programmable components for gene editing and nucleic acid detection. Notably, assays such as SHERLOCK and DETECTR leverage these guide RNAs to identify viral sequences with exceptional sensitivity, revolutionizing pathogen detection, exemplified during the COVID-19 pandemic.
| Relevant Category | Substantive Data |
|---|---|
| Sensitivity | Detection limits as low as single molecule per microliter in advanced biosensors |
| Speed | Results obtainable within 30 minutes, facilitating point-of-care diagnostics |
| Specificity | High discriminative capacity due to sequence-specific hybridization, with mismatch discrimination over 99% |
Nanotechnology and Material Science
Programmable nucleic acids serve as building blocks in DNA origami and nanostructures, enabling precise spatial arrangement of molecules at the nanoscale. These constructs find applications in drug delivery, biosensing, and the creation of molecular machines. Researchers have demonstrated DNA-based nanorobots capable of targeted payload delivery within cellular environments, a testament to the innovative potential of nucleic acid programming.
Moreover, the integration of nucleic acids with inorganic nanomaterials has led to the development of hybrid devices with enhanced optical, electronic, and catalytic properties, fostering innovations in biosensors and energy harvesting systems.
Key Points
- Polymeric nucleic acids enable targeted, sequence-specific gene regulation and therapy, with chemical modifications improving efficacy
- Innovations like LNPs and CRISPR-based detection systems have transformed diagnostics and therapeutics, exemplifying rapid translation from bench to bedside
- DNA nanostructures expand the horizons of nanomedicine, biosensing, and programmable materials, showcasing the intersection of biochemistry with material science
- Global market projections estimate nucleic acid therapeutics reaching over $10 billion by 2027, reflecting growing industrial relevance
- Continuous evolution of synthesis, delivery, and modification techniques drives the next wave of innovation in the field
Emerging Innovations and Future Directions
As research accelerates, several groundbreaking innovations are poised to redefine the scope and impact of polymeric nucleic acids. CRISPR-Cas12 and Cas13 variants expand the capabilities for gene editing and nucleic acid detection, including RNA targeting and multiplexed diagnostics. The development of self-assembling DNA origami structures with functional elements embedded promises dynamic nanodevices capable of responding to environmental stimuli.
The convergence of artificial intelligence and nucleic acid chemistry facilitates the design of smarter, more efficient therapeutic agents, culminating in personalized nanomedicine. Additionally, the integration of nucleic acids with biomimetic materials is fostering new horizons in tissue engineering and regenerative medicine.
What are the most recent breakthroughs in nucleic acid therapeutics?
+Recent breakthroughs include the approval of mRNA vaccines leveraging lipid nanoparticle delivery systems, and the development of chemically modified oligonucleotides that exhibit enhanced stability and tissue-specific targeting. Innovations in delivery vehicles—such as exosomes and targeted nanoparticles—are further improving therapeutic index.
How are synthetic nucleic acids advancing diagnostic technology?
+Synthetic nucleic acids underpin highly sensitive assays like CRISPR-based detection systems (e.g., SHERLOCK), which enable real-time, point-of-care diagnosis with minimal equipment. The customization and chemical stabilization of these molecules significantly improve assay performance and shelf-life, broadening their applicability in diverse healthcare settings.
What challenges remain in the field of nucleic acid nanotechnology?
+Key challenges include achieving precise control over nucleic acid self-assembly at scale, ensuring in vivo stability, and developing targeted, efficient delivery methods. Overcoming immunogenicity and off-target effects also remain critical considerations for clinical translation.